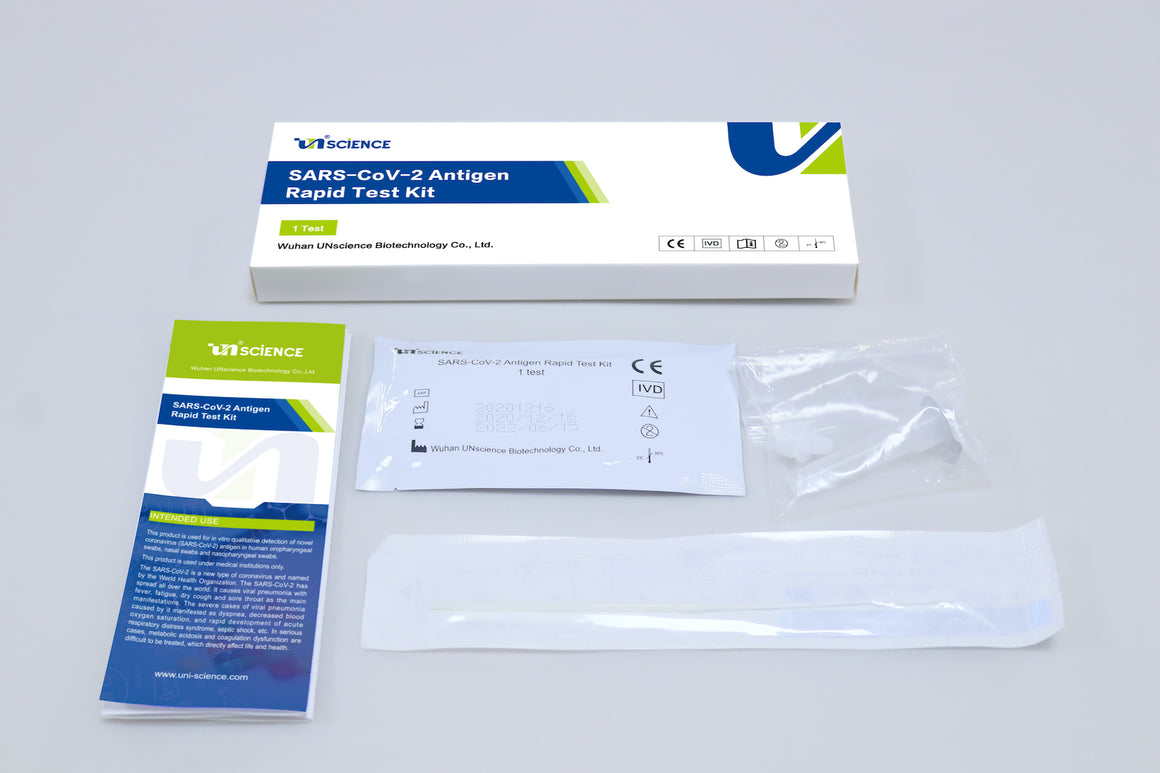
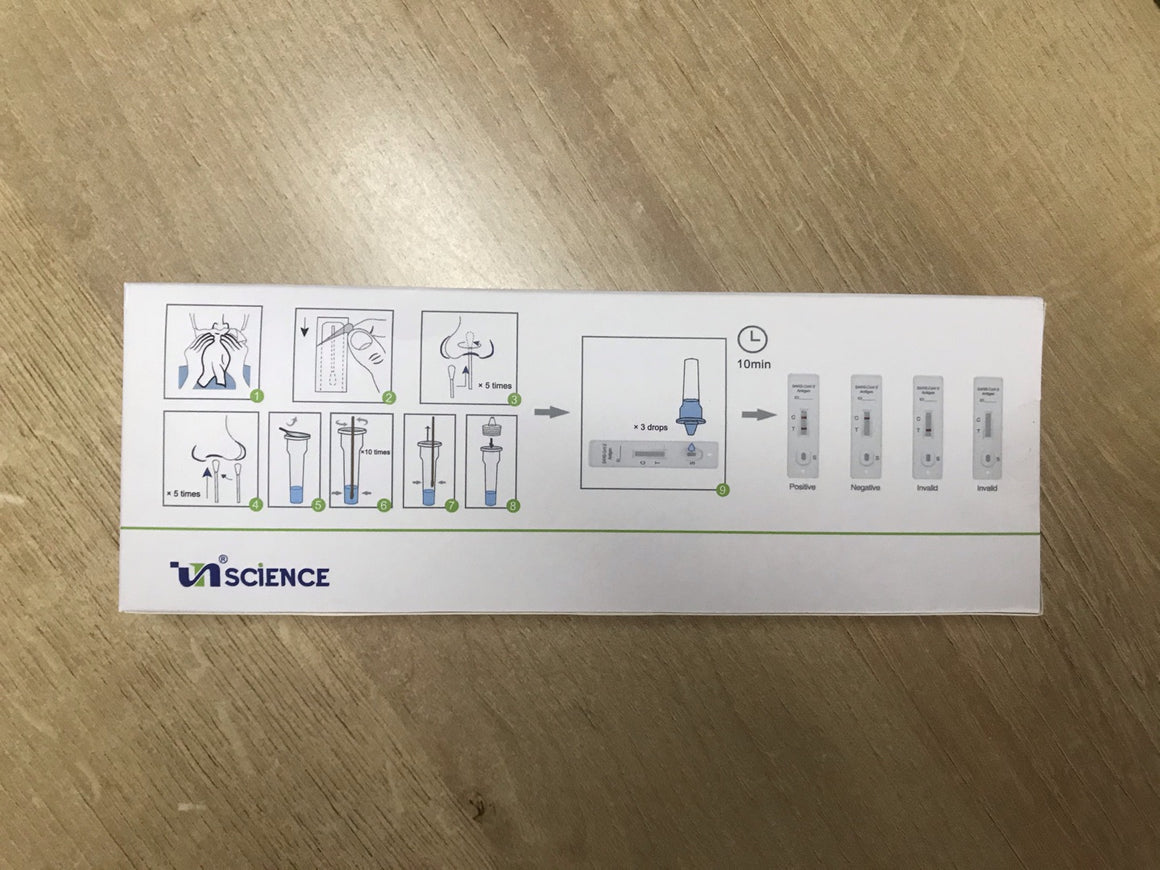
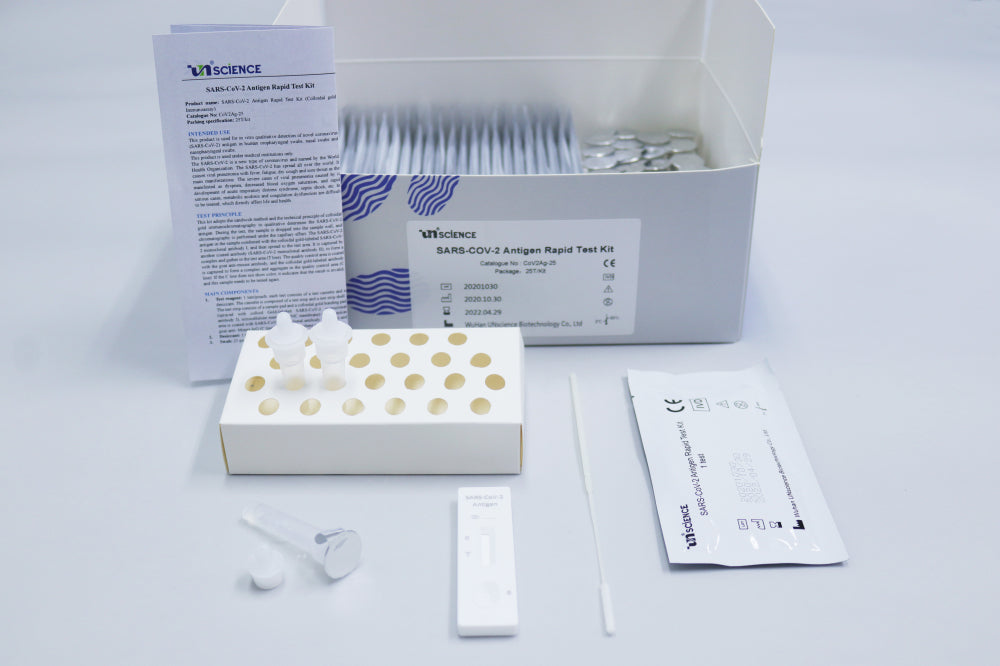
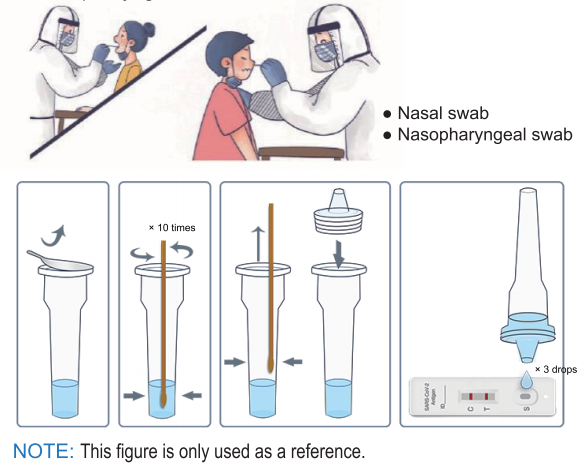
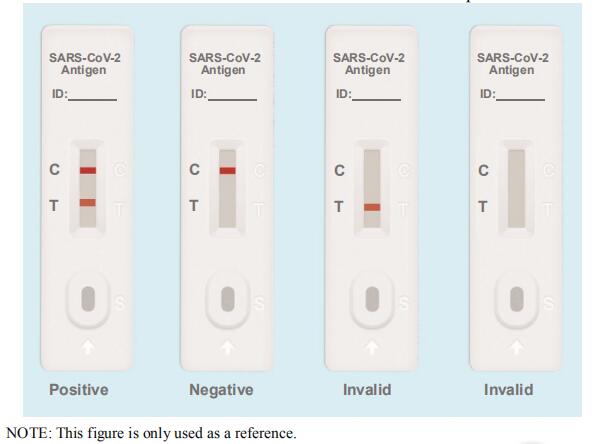
Limitations of SARS-CoV-2 Antigen Rapid Test Kit
1.This kit is a qualitative test for in vitro auxiliary diagnosis.
2.Due to methodological limitations, the sensitivity of this kit is lower than that of PCR. Therefore, more attention should be paid to the negative results of this experiment, and a comprehensive judgment should be combined with other test results. It is recommended that the suspected results be supplemented with nucleic acid testing or virus isolation and culture in vitro for confirmation.
3.Unreasonable sampling, transportation and handling, or low virus content in the sample will lead to false negative results.
4.The test results of this reagent are for clinical reference only and cannot be used as the only basis for clinical diagnosis. The tester should conduct a comprehensive evaluation based on the patient's clinical manifestations and other laboratory test results.
About UNscience
UNscience a wholly owned subsidiary of Elabscience, specializes in the research and development, production and sale of in-vitro diagnostic reagents. Certified with ISO 13485 and other documents, UNscience sell its products all over the world.
The company has 100,000 grade GMP purification workshop and quality management system, three major technical platforms (Colloidal Gold Immunochromatographic Platform, Fluorescence Immunochromatographic Platform, and Pathological Diagnosis Antibody Platform). UNscience has the independent research and development and production capacity of core raw materials, and has successfully developed 27 POCT immunochromatographic quantitative detection products (colloidal gold and fluorescence), mainly covering cardiovascular and cerebrovascular diseases, kidney diseases, diabetes, infectious diseases, reproductive health, health examination and other.